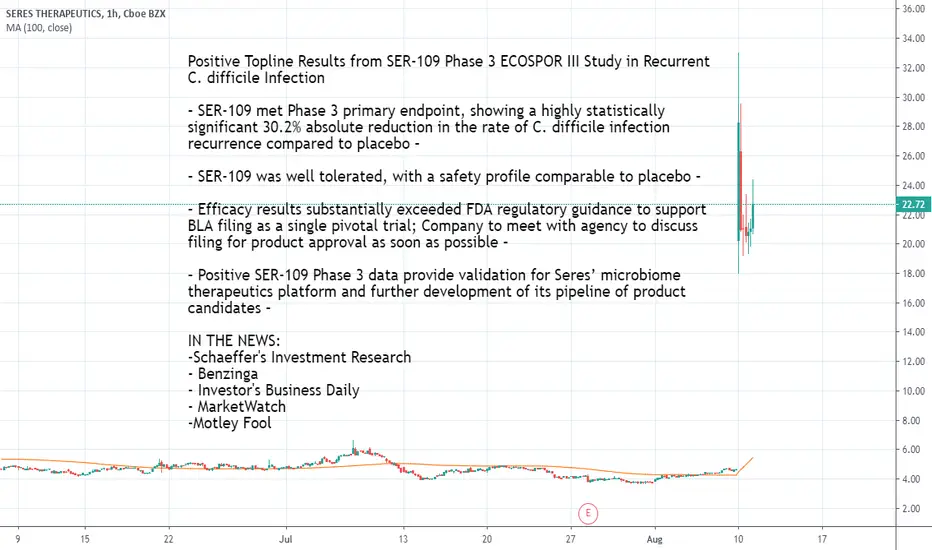
Positive Topline Results from SER-109 Phase 3 ECOSPOR III Study in Recurrent C. difficile Infection
– SER-109 met Phase 3 primary endpoint, showing a highly statistically significant 30.2% absolute reduction in the rate of C. difficile infection recurrence compared to placebo –
– SER-109 was well tolerated, with a safety profile comparable to placebo –
– Efficacy results substantially exceeded FDA regulatory guidance to support BLA filing as a single pivotal trial; Company to meet with agency to discuss filing for product approval as soon as possible –
– Positive SER-109 Phase 3 data provide validation for Seres’ microbiome therapeutics platform and further development of its pipeline of product candidates –
IN THE NEWS:
-Schaeffer's Investment Research
- Benzinga
- Investor's Business Daily
- MarketWatch
-Motley Fool
finance.yahoo.com/news/seres-therapeutics-announces-positive-topline-103000869.html?guce_referrer=aHR0cHM6Ly9maW52aXouY29tL3F1b3RlLmFzaHg_dD1NQ1JCJnR5PWMmcD1kJmI9MQ&guce_referrer_sig=AQAAAE9w_E6Ku0HueBDtCrh4go-VOSqkBrTcmTwKxhzcdOVKPExn5qBDCPxCJSOB3-cXR1Z_IB6IwbBZmL4tu63628Outd7xSvt1h8PWMCk4XopvYnvp_RfZl82mwVfil4rNffHUSoLMmnE6W69qL5tI6yh-UOcevUmfiZBZmMhUqssn&_guc_consent_skip=1597092504
– SER-109 met Phase 3 primary endpoint, showing a highly statistically significant 30.2% absolute reduction in the rate of C. difficile infection recurrence compared to placebo –
– SER-109 was well tolerated, with a safety profile comparable to placebo –
– Efficacy results substantially exceeded FDA regulatory guidance to support BLA filing as a single pivotal trial; Company to meet with agency to discuss filing for product approval as soon as possible –
– Positive SER-109 Phase 3 data provide validation for Seres’ microbiome therapeutics platform and further development of its pipeline of product candidates –
IN THE NEWS:
-Schaeffer's Investment Research
- Benzinga
- Investor's Business Daily
- MarketWatch
-Motley Fool
finance.yahoo.com/news/seres-therapeutics-announces-positive-topline-103000869.html?guce_referrer=aHR0cHM6Ly9maW52aXouY29tL3F1b3RlLmFzaHg_dD1NQ1JCJnR5PWMmcD1kJmI9MQ&guce_referrer_sig=AQAAAE9w_E6Ku0HueBDtCrh4go-VOSqkBrTcmTwKxhzcdOVKPExn5qBDCPxCJSOB3-cXR1Z_IB6IwbBZmL4tu63628Outd7xSvt1h8PWMCk4XopvYnvp_RfZl82mwVfil4rNffHUSoLMmnE6W69qL5tI6yh-UOcevUmfiZBZmMhUqssn&_guc_consent_skip=1597092504
Declinazione di responsabilità
Le informazioni ed i contenuti pubblicati non costituiscono in alcun modo una sollecitazione ad investire o ad operare nei mercati finanziari. Non sono inoltre fornite o supportate da TradingView. Maggiori dettagli nelle Condizioni d'uso.
Declinazione di responsabilità
Le informazioni ed i contenuti pubblicati non costituiscono in alcun modo una sollecitazione ad investire o ad operare nei mercati finanziari. Non sono inoltre fornite o supportate da TradingView. Maggiori dettagli nelle Condizioni d'uso.