Merck & Co. (NYSE:  MRK) traded lower at $101.53 after receiving conditional FDA approval for two new cattle antiparasitic treatments—Exzolt Cattle-CA1 and Dectomax-CA1. The approvals target rising cases of New World screwworm larvae and fever ticks, two livestock threats that the FDA says could drive hundreds of millions in economic losses. The muted stock reaction reflects broader market caution rather than concern over Merck’s fundamentals.
MRK) traded lower at $101.53 after receiving conditional FDA approval for two new cattle antiparasitic treatments—Exzolt Cattle-CA1 and Dectomax-CA1. The approvals target rising cases of New World screwworm larvae and fever ticks, two livestock threats that the FDA says could drive hundreds of millions in economic losses. The muted stock reaction reflects broader market caution rather than concern over Merck’s fundamentals.
Exzolt Cattle-CA1 is a pour-on solution designed to disrupt parasite growth, while Dectomax-CA1 uses the same active ingredient as fully approved Dectomax, allowing regulators to clear the drug without new safety or manufacturing submissions. The updated formula offers 21 days of reinfestation protection and maintains the same 35-day withdrawal period for treated cattle. The FDA highlighted the urgency of getting these solutions to producers as livestock risks rise heading into 2026.
Despite the regulatory win, Merck continues to underperform major indices. The stock is up just 5% YTD versus the S&P 500’s 16.57%, and the three-year return of 0.91% trails the index’s 68.38%. Still, Merck’s animal health segment remains an important revenue pillar and positions the company competitively as agricultural threats increase.
Technical Analysis
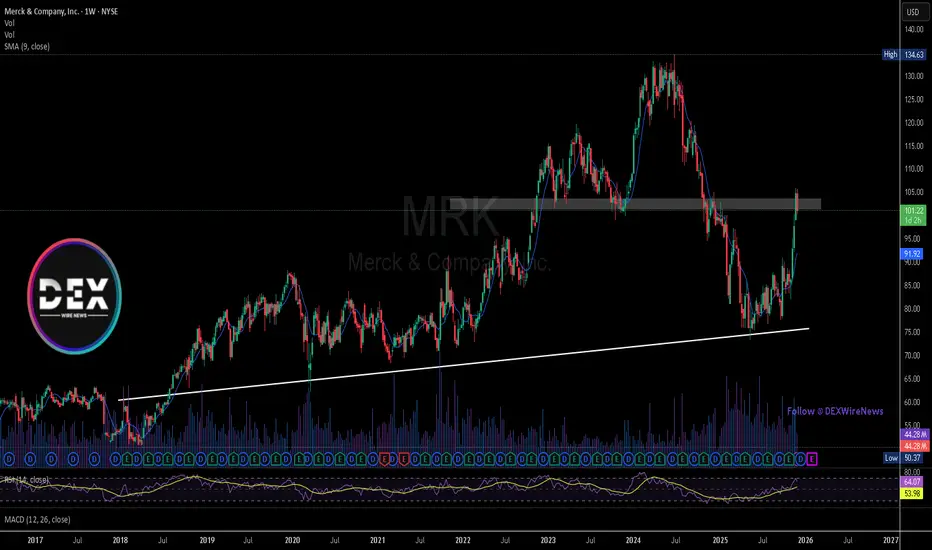
The weekly chart shows MRK rallying strongly from long-term trendline support near $72–$75, reclaiming the $95 level and tapping into a major supply/rejection zone around $102. Price reacted sharply from that area, confirming it as resistance. A clean breakout above $102 could open a path toward $134, where the next structural high sits. If sellers remain active, support lies at $95, with deeper structure at $72.
MRK rallying strongly from long-term trendline support near $72–$75, reclaiming the $95 level and tapping into a major supply/rejection zone around $102. Price reacted sharply from that area, confirming it as resistance. A clean breakout above $102 could open a path toward $134, where the next structural high sits. If sellers remain active, support lies at $95, with deeper structure at $72.
Exzolt Cattle-CA1 is a pour-on solution designed to disrupt parasite growth, while Dectomax-CA1 uses the same active ingredient as fully approved Dectomax, allowing regulators to clear the drug without new safety or manufacturing submissions. The updated formula offers 21 days of reinfestation protection and maintains the same 35-day withdrawal period for treated cattle. The FDA highlighted the urgency of getting these solutions to producers as livestock risks rise heading into 2026.
Despite the regulatory win, Merck continues to underperform major indices. The stock is up just 5% YTD versus the S&P 500’s 16.57%, and the three-year return of 0.91% trails the index’s 68.38%. Still, Merck’s animal health segment remains an important revenue pillar and positions the company competitively as agricultural threats increase.
Technical Analysis
The weekly chart shows
⭐⭐⭐ Sign Up for Free ⭐⭐⭐
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Chat Room >> discord.gg/x3EQven3ZY
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Chat Room >> discord.gg/x3EQven3ZY
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
Pubblicazioni correlate
Declinazione di responsabilità
Le informazioni e le pubblicazioni non sono intese come, e non costituiscono, consulenza o raccomandazioni finanziarie, di investimento, di trading o di altro tipo fornite o approvate da TradingView. Per ulteriori informazioni, consultare i Termini di utilizzo.
⭐⭐⭐ Sign Up for Free ⭐⭐⭐
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Chat Room >> discord.gg/x3EQven3ZY
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
1) Download our Mobile App >> link-to.app/dexwirenews
2) Join our Chat Room >> discord.gg/x3EQven3ZY
3) Sign Up for Text Alerts >>
dexwirenews.com/TEXT
4) Follow @DEXWireNews on Social Media
Pubblicazioni correlate
Declinazione di responsabilità
Le informazioni e le pubblicazioni non sono intese come, e non costituiscono, consulenza o raccomandazioni finanziarie, di investimento, di trading o di altro tipo fornite o approvate da TradingView. Per ulteriori informazioni, consultare i Termini di utilizzo.